health
February 14, 2026
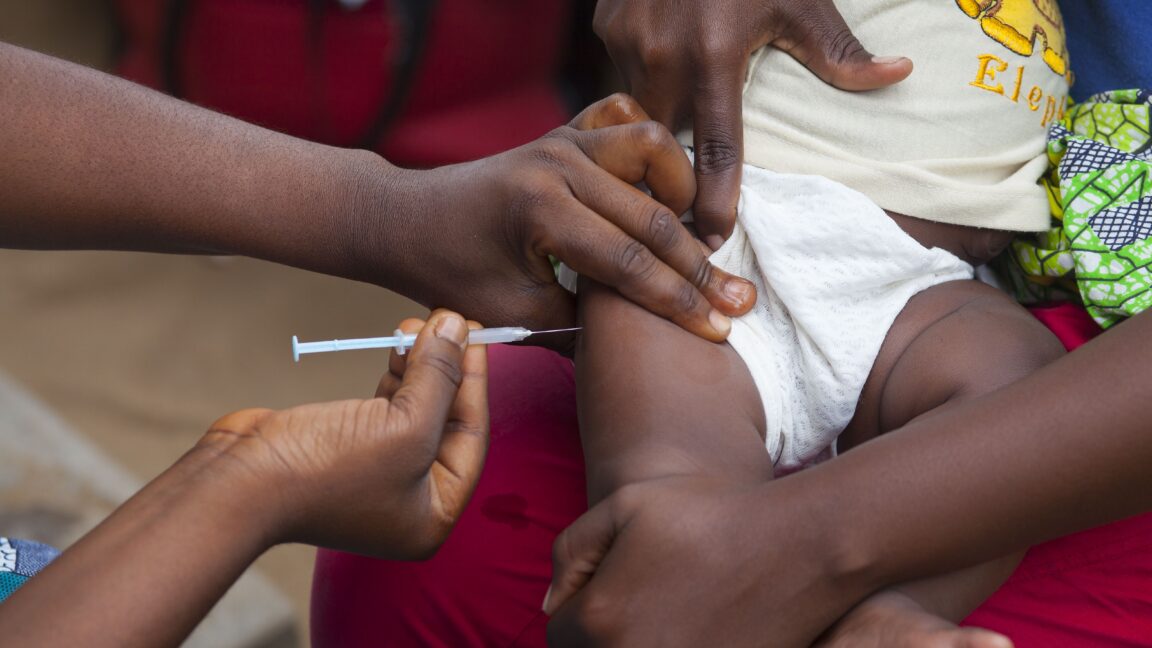
WHO slams US-funded newborn vaccine trial as "unethical"
CDC awarded $1.6 million for study birth dose of hepatitis B vaccine in Guinea-Bissau.
TL;DR
- The WHO has formally stated a US-funded vaccine trial is unethical.
- The trial withholds a hepatitis B vaccine birth dose from some newborns in Guinea-Bissau.
- The Centers for Disease Control and Prevention (CDC) provided $1.6 million for the trial.
- Danish researchers Christine Stabell Benn and Peter Aaby are leading the trial.
- The WHO cited concerns about harm reduction, benefit to participants, and bias in the trial's design.
- Withholding the vaccine could lead to serious and irreversible harm like chronic infection, cirrhosis, and liver cancer.
- The trial appears to be suspended pending reviews, though the US Department of Health and Human Services initially stated it was proceeding.
- The hepatitis B vaccine birth dose is an essential public health intervention that prevents life-threatening liver disease.
Continue reading
the original article